German startup to market first artificial iris

The first artificial iris -- the colored part of the eye that surrounds the pupil -- is cleared for use in the U.S.
The Food and Drug Administration on Wednesday gave Germany's HumanOptics the go-ahead to market its device for use in adults and children whose iris is missing due to a congenital condition called aniridia or because of an injury.
A rare genetic disorder, aniridia occurs in about one in 50,000 to 100,000 newborns worldwide, according to the National Institutes of Health. Those with the condition suffer from vision problems including a sensitivity to light because the iris controls the amount of light coming into the eye.
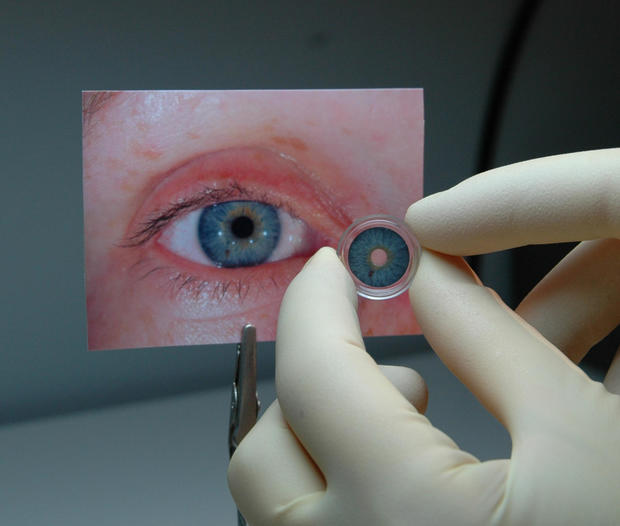
"Patients with iris defects may experience severe vision problems, as well as dissatisfaction with the appearance of their eye," Dr. Malvina Eydelman, who directs the division of ophthalmic, and ear, nose and throat devices at the FDA's Center for Devices and Radiological Health, said in a news release.
The "approval of the first artificial iris provides a novel method to treat iris defects that reduces sensitivity to bright light and glare. It also improves the cosmetic appearance of the eye in patients with aniridia," she added.

In addition to congenital aniridia, the CustomFlex Artificial Iris can be used to treat iris defects due to other reasons or conditions, such as albinism, traumatic injury or surgical removal due to melanoma, the FDA said.
The surgically implanted prosthetic is made of thin, foldable silicone and is sized and colored for individual patients, according to the agency. It was shown to be safe and effective in a clinical trial of 389 adults and children with aniridia or other iris defects.
- In:
- Food and Drug Administration
Disclaimer: The copyright of this article belongs to the original author. Reposting this article is solely for the purpose of information dissemination and does not constitute any investment advice. If there is any infringement, please contact us immediately. We will make corrections or deletions as necessary. Thank you.







